- Product Details
Keywords
- Tenofovir alafenamide
- 99.5% Tenofovir alafenamide
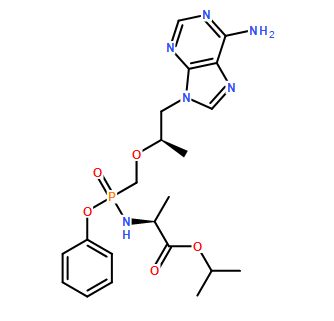
- C21H29N6O5P
Quick Details
- ProName: CAS NO.379270-37-8 High Purity Tenofov...
- CasNo: 379270-37-8
- Molecular Formula: C21H29N6O5P
- Appearance: White powder
- Application: Pharmaceutical Intermediates
- DeliveryTime: 5-7 Business days
- PackAge: 10g; 100g;1kg; 25kg
- Port: Tianjin China
- ProductionCapacity: 200 Kilogram/Week
- Purity: 99.5%
- Storage: Keep in dry and cool condition
- Transportation: by air or by sea
- LimitNum: 10 Gram
Superiority
AllBest (Tianjin AllBest Technology Co., Ltd.) is a professional manufacturer and research institute of APIs (Active Pharmaceutical Ingredients) and Pharmaceutical intermediates. Our products and services are served to customers and partners around the world.
AllBest establish in Tianjin, P.R.China, in 2011. And we build our own research center after then. We are capable of reseach and improve all main APIs & Pharmaceutical intermediates production flows. Experts are one of our competitiveness. Beside we have built strong partnership with many university laboratories also the researcher of laboratories. Therefore we are capable of providing world leading products and solutions.
Details
Tenofovir alafenamide (TAF) is an investigational oral prodrug of the HIV-1 nucleotide reverse transcriptase inhibitor tenofovir (TFV). Tenofovir disoproxil fumarate (TDF) is another TFV prodrug, widely used for the treatment of HIV-1 infection. TAF is converted mostly intracellularly to TFV and, in comparison to TDF, achieves higher tenofovir diphosphate (TFV-DP) levels in peripheral blood mononuclear cells.
Molecular Formula
Certificate of Analysis
| Product Name: Tenofovir Alafenamide (GS-7340) | Batch No.: BOZ/TAF/17/Mar/03 |
| CAS No.: 379270-37-8 | Quantity: 100grms |
| Manufacturing Date: Mar., 3rd, 2017 | Pack Size: 100*1pack |
| Text Date: Mar., 3rd, 2017 | Country of Origin: China |
| Expiry Date: Mar., 2nd, 2018 | Quality Standard: Enterprise Standard |
| TEST | SPECIFICATION | RESULT |
| Physical Characters | ||
| Appearance | White to off-white powder | Off-white powder |
| Identification | HPLC: Sample and standard exhinit the same retention tiem | Conforms |
| Impurities | ||
| Related Substance |
Impurity A: ≤0.15% Impurity B: ≤0.15% Any other individual impurity: ≤0.15% Total impurities: ≤0.15% |
0.04% 0.03% 0.04% 0.3% |
| Chemical Characters | ||
| Heavy Metals | ≤20PPM | Conforms |
| Solvent residues |
Methanol: ≤3000ppm Toluene: ≤8900ppm Acetone: ≤5000ppm |
1900ppm 410ppm 2600ppm |
| Water | ≤1.0% | 0.4% |
| Assay (by HPLC) | 98.0%-102.0% | 99.6% |
|
Packing - Aluminum foil bag. Storage - Store in well closed container. |
||
Conclusion: The above analysis results comply with the specification. Customer is advised to verity the results before use.





